Effects of precursors composition on characteristics of organic-inorganic Methyl Ammonium Lead Trihalide Perovskite-based thin films
Keywords:
Mixed halide perovskites, organo-metallic perovskites, energy bandgaps, crystallites, morphological properties, mixed compositionAbstract
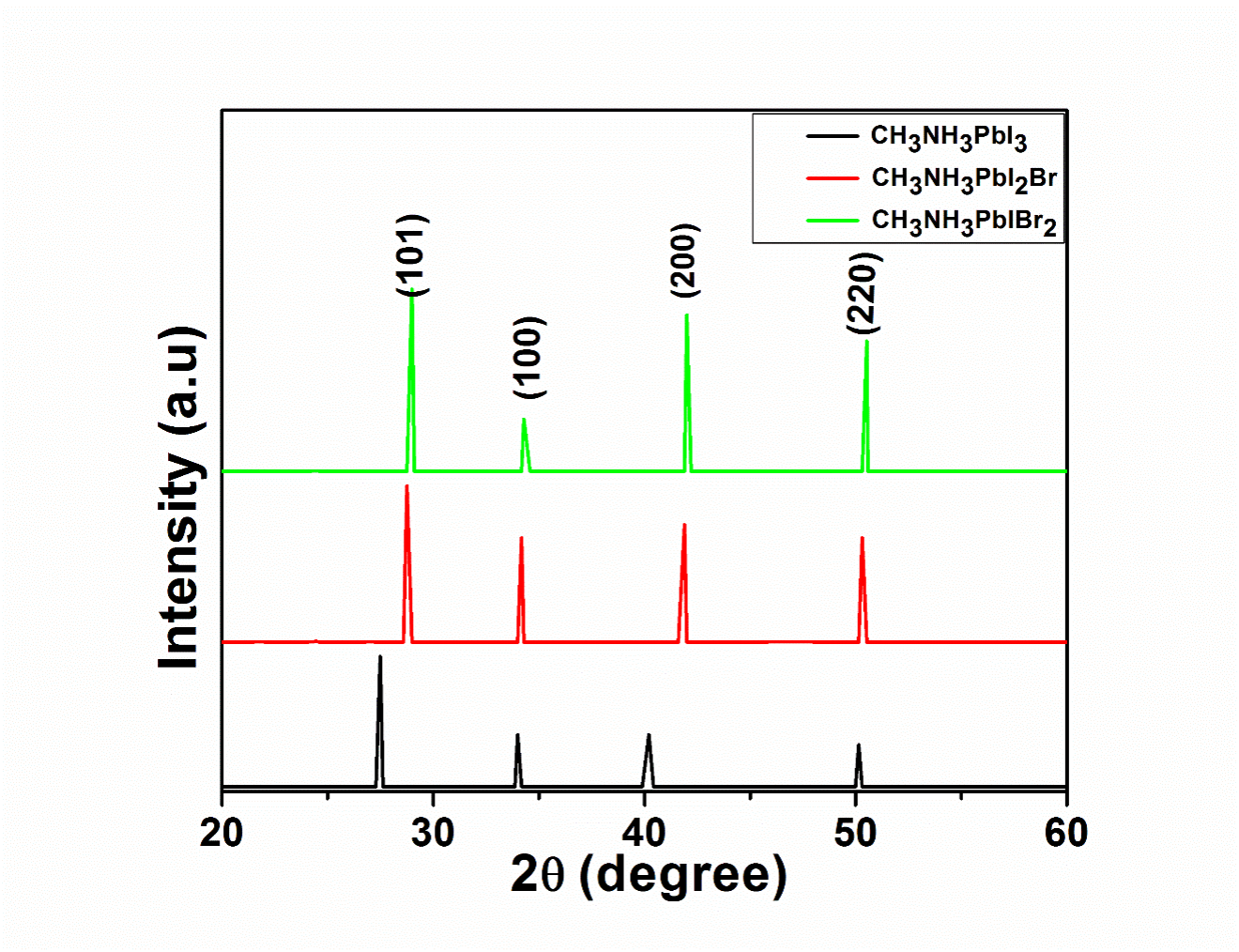
Precursors compositional engineering is considered as one of the viable methods of manipulating the characteristics of the mixed halide perovskites. In this research, methyl ammonium lead triiodide perovskites (CH3NH3PbI3) was synthesized through solution-based processing and deposited on ITO glass substrates using spin coating method. Additionally, the effects of mixed composition of precursors on the deposited films were evaluated by partial replacement of iodine (I) anion with bromine (Br) on CH3NH3Pb(I1−xBrx)3 perovskite with Br compositions for x = 0.33 and x = 0.66. The deposited films were characterized using X-ray Diffraction (XRD), Fourier Transform Infrared (FTIR) Spectrometry, Ultraviolet-visible Spectrophotometry, Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM) and Solar Simulator to study the structural, optical, morphological and optoelectronic properties of the films. The average crystallites of ∼7.94, ∼7.70 and ∼7.62 nm were calculated for CH3NH3PbI3, methyl ammonium lead diiodide bromide (CH3NH3PbI2Br) and methyl ammonium lead iodide dibromide (CH3NH3PbIBr2) respectively. In addition, the energy bandgaps of 1.63 eV, 1.91 eV and 1.94 eV were extrapolated for CH3NH3PbI3, CH3NH3PbI2Br and CH3NH3PbBr2I respectively. The results from J-V curves show the Jsc values of 9.9, 9.2 and 8.6 mAcm−2, FF values of 67.00, 58.20 and 54.2%, Voc values of 0.55, 0.54 and 0.52, and PCE values of 3.50, 3.33 and 3.00% were calculated for CH3NH3PbI3, CH3NH3PbI2Br and CH3NH3PbBr2I, respectively. The results show that partial replacement of I anion with Br on CH3NH3Pb(I1−xBrx)3 perovskite with Br compositions for x = 0.33 and x = 0.66 did not improve the functionality of the methyl ammonium lead triiodide perovskite material.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Simeon Amole, Oluwaseun Adedokun, Olusola Akinrinola, Olumuyiwa Aderemi Oyekanmi, Festus Akintunde Ojeniyi, Adekunle Kazeem Dauda, Ayodeji Oladiran Awodugba

This work is licensed under a Creative Commons Attribution 4.0 International License.



