Role of Van der Waals correction on the catalytic performance of 1T-TiS₂ electrocatalyst
Keywords:
Vander Waals interactions, Catalytic performance, Density functional theory, Adsorbed hydrogenAbstract
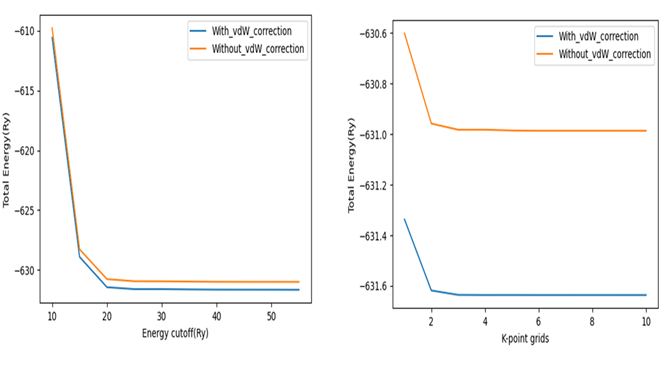
In this paper, the role of Van der Waals (vdW) correction on the catalytic performance of 1T- TiS2 material was investigated within the framework of density functional theory (DFT) and dispersion-corrected density functional theory (DFT-D3). The exchange-correlation functional was approximated using generalized gradient approximation (GGA) as parameterized by Perdew-Burke-Ernzerhof (PBE). Based on our results, the calculated lattice parameters were a =3.33 Å and a =3.32 Å upon calculations without and with inclusion of vdW correction, respectively, which indicated a slight reduction of ∼ 0.3% when vdW correction was included. In both cases, the value of a was in good agreement with previous experimental and theoretical data. However, the distances between the adsorbed hydrogen (H) and the surface of the catalyst were affected by the vdW correction. Our findings also showed that the vdW correction has an impact on the catalytic performance of 1T-TiS2. The Gibbs free energy change for hydrogen adsorption (ΔGH∗) calculated for the most stable adsorption location was ∼ - 0.55 eV and ∼ - 0.53 eV without and with vdW correction, respectively. This revealed that the one calculated with the inclusion of vdW correction is closer to the optimal value. Therefore, this emphasized the need of including a vdW correction in any DFT study that involves catalytic properties of this material and its related members for more accurate and reliable results.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Shamsuddeen Sani Alhassan, Mahmud Abdulsalam, Abdullahi Tanimu, Ibrahim Muhammad Bagudo

This work is licensed under a Creative Commons Attribution 4.0 International License.



