Attenuation of indomethacin-induced gastric ulceration by methanolic extract of Cucumis Melo (L. indorous) seeds in male Wistar rats
Keywords:
Indomethacin, Ulceration, Catalase, TNF-A, Nitrite levelAbstract
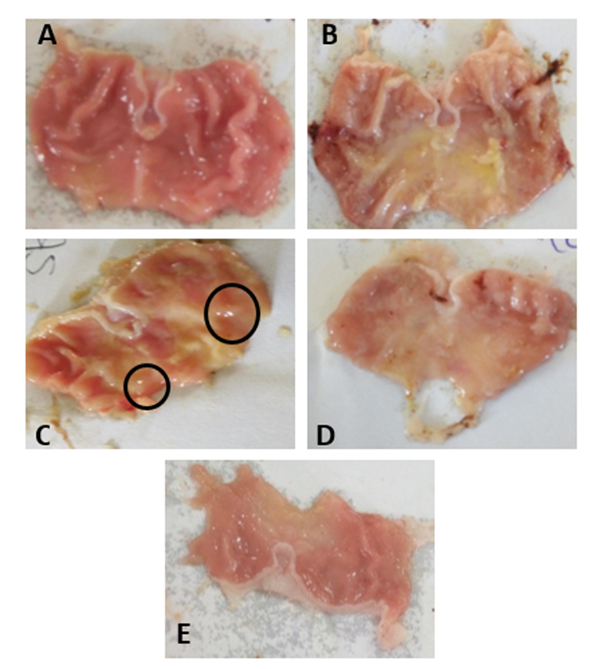
Inflammatory markers has been implicated during NSAID-induced gastric ulcer as it enhances leukocyte adhesion which also contribute to lipid peroxidation and decrease in antioxidants. Cucumis melo seeds (L. Indorous) is well known to contain some active component with strong anti-inflammatory potentials. Therefore, this study aimed to investigate the effects of Cucumis melo seed extract (MECmS) on indomethacin-induced stomach ulcers in male Wistar rats. Twenty-five male Wistar rats (n = 5, 130–150 mg) were randomly assigned to five groups: group 1 (water); group 2 (indomethacin; 40 mg/Kg); group 3 (50 mg/Kg methanolic extract of Cucumis melo seeds; MECmS + indomethacin); group 4 (100 mg/kg MECmS + indomethacin); and group 5 (200 mg/kg MECmS + indomethacin). MECmS was administered 14 days prior to induction of ulcers. Organs’ relative weights (RWO), total stomach acidity, ulcer score, and hematological parameters were assessed. MDA, catalase, protein level, nitrite, and TNF-alpha were also measured. Induction with indomethacin led to significant (p<0.05) increase in gastric acidity, ulcer score, relative stomach weight, MDA, TNF-alpha, and significant decrease in total protein, catalase, and nitrite level. Indomethacin also induced significant necrosis of the tunic mucosal. Treatment with Cucumis melo seed extract reversed tunic mucosal necrosis and significantly induced the proliferation of the gastric mucosal gland. Cucumis melo seed extract also significantly increased total protein, catalase, and nitrite level, while it significantly reduced ulcer score, gastric acidity, MDA and TNF-alpha levels. Cucumis melo possess antiulcer and anti-inflammatory properties; hence can be explored as a novel anti-ulcer drug.
Published
How to Cite
Issue
Section
Copyright (c) 2024 Grace Adebayo-Gege, James Adiwu, Abednego Ovey Angbashim, Toyin Dorcas Alabi, Murtala Ngabea Audu , Frank Abimbola Ogundolie

This work is licensed under a Creative Commons Attribution 4.0 International License.



