Temperature-dependent viscometry of baobab pectin (Adansonia digitata L.)
Keywords:
Baobab pectin, Viscosity-temperature dependency, Arrhenius parameters, Thermodynamic parametersAbstract
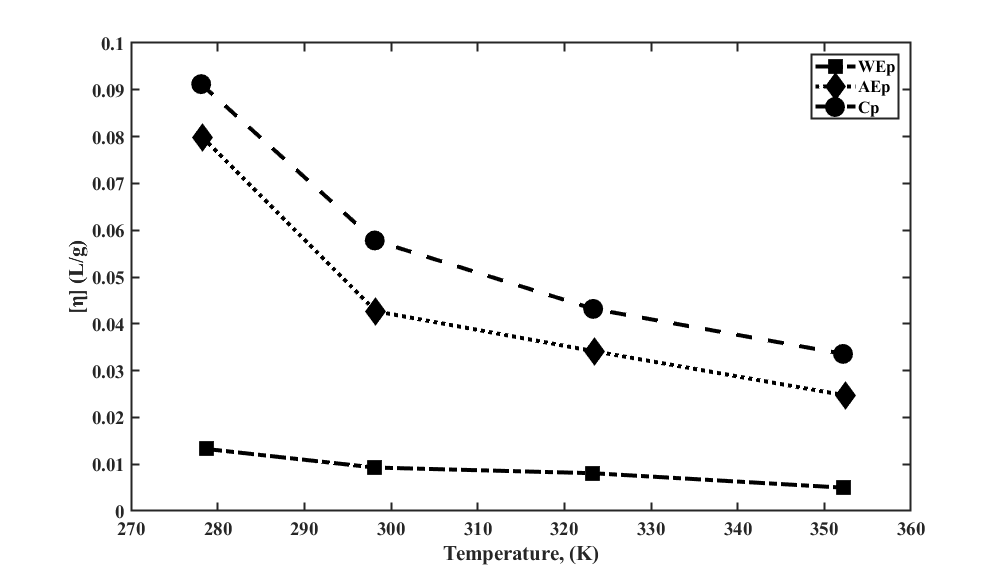
This study uses the Arrhenius-type equation and the Frenkel-Eyring equation to evaluate the viscosity-temperature dependency of the baobab pectin (BoP) solution at 278.16-353.16 K. The viscosity parameters (apparent viscosity, ηA and intrinsic viscosity, [η]) were analysed for polymeric systems of WEp (pectin extracted using water), AEp (pectin extracted using acid), and Cp (citrus pectin, a control). A Vibro-viscometer was used to measure ηA, while [η] values were estimated from the Kuwara equation. The viscosity parameters decrease with an increase in temperature, though the effects were more pronounced in WEp than in AEp and Cp. Data from [η] indicated that activation energy Ea was higher for AEp (12.24 kJ/mol) and lower for WEp (10.18 kJ/mol). In contrast, the ηA data had a higher Ea for WEp (21.38 kJ/mol) and a lower Ea for Cp (16.49 kJ/mol). The ηA data showed a non-linear viscosity-temperature relationship, and the Vogel-Fulcher-Tammann-Hesse equation was used instead to relate the temperature dependency of BoP. The flow patterns of all pectin solutions showed positive entropy (ΔS + v ), positive enthalpy (ΔH+ v ), and negative Gibbs free energy (ΔG−v ). This revealed that the flow was disordered, dependent on temperature, and spontaneous. Overall, the viscous flow of WEp was more sensitive and less dependent on temperature compared to AEp and Cp.
Published
How to Cite
Issue
Section
Copyright (c) 2024 Shadreck Muyambo, Jack A. Urombo

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Most read articles by the same author(s)
- Shadreck Muyambo, Jack A Urombo, Garry K. Masoha, Livingstone Jenya, A mathematical framework for assessing food fraud intentions , African Scientific Reports: Volume 4, Issue 3, December 2025



